Hair thickness tests are false

How to cite: Wong M. Hair porosity test is false. Lab Muffin Beauty Science. January 28, 2026. Accessed January 28, 2026.
There are many hair care tips based on the idea of ”hair porosity”. This is a pretty complex word that can mean many different things in different contexts (this may be a long long in-depth one another day!). A lot of advice leads people to products that suit their hair better, but this is just a coincidence – many ideas about porosity don’t make a lot of sense.
This article is adapted from my video about hair extensions. See also Part 1 on hair and water in more detailand Part 2 of the myth of hygral exhaustion.
Undamaged and well-groomed hair is waterproof
Most of the myths surrounding porosity come from the misconception that hair is impermeable to water: in undamaged hair, the cuticle is thought to seal in water, while conditioners mimic the function of damaged hair. This is not true!
Undamaged hair can absorb about 30% (about one-third) of its weight in water, within minutes. The water in undamaged, conditioned hair also changes quickly, depending on the humidity.
| Relative humidity (%) | Weight of water absorbed (%) |
|---|---|
| 0 | 0 |
| 8 | 3.9 |
| 40 | 10.2 |
| 63 | 14.8 |
| 86 | 22.6 |
| 100 | 31.2 |
From Robbins CR. Chemical and Physical Behavior of Human Hair. Springer Berlin Heidelberg 2012.
This is because the natural F layer that repairs the hair is only above the cuticle scale – there are many gaps where water can enter (hair is very similar to pine).
Conditioners are also waterproof. They apply blobs, not one continuous layer.
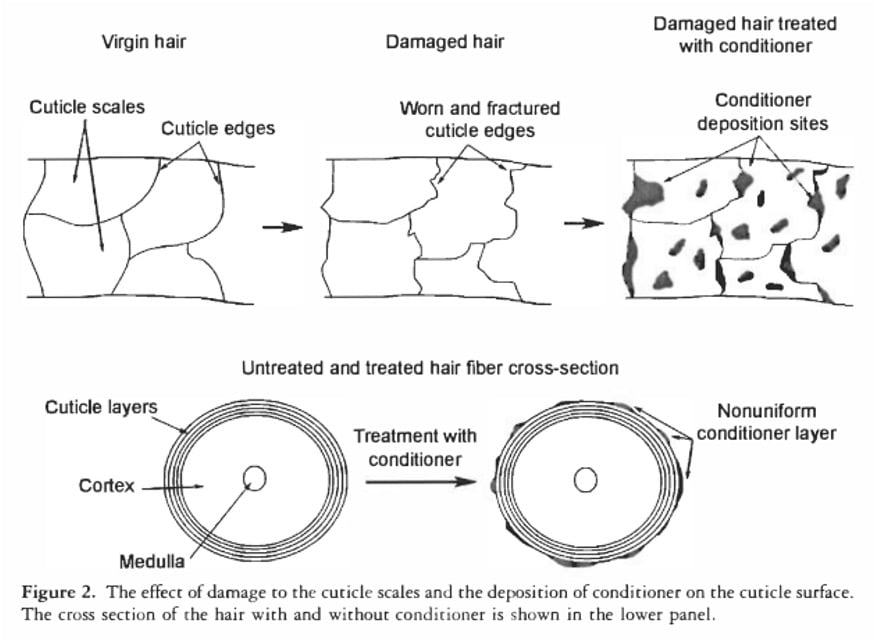

The conditioner blobs are small enough to make hair feel smooth and soft to the touch. But individual water molecules are small, so conditioner blobs are less effective at keeping water out.
“Porosity Test”
This can be confusing if you’ve seen tests that claim to diagnose your hair. Examples:
Float test: Put a strand of hair in a glass of water. Heavily damaged hair sinks, while lightly damaged hair floats. This is described as “very strong hair” that sinks when it absorbs enough water.


Drop Test: Put a drop of water on the lock of hair. In undamaged hair it stays in fine round beads, but it spreads out in damaged hair. This is explained by the water entering the “high porosity” hair because it is full of holes.


If undamaged hair doesn’t hold water, what happens?
The answer is local tension. What you notice is actually related to it the way water interacts with the surface of the hair,no even if it goes inside!
What is surface tension?
In liquid water, individual water molecules really like to hold hairs together through hydrogen bonds. The water molecules on the surface have smaller neighbors that they can hold hands with, so it’s like they have more hands on the surface.
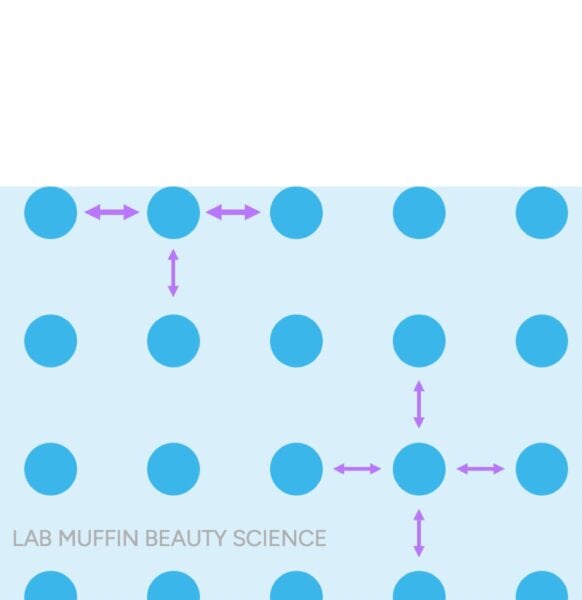

This creates a kind of tough “skin” that can trap insects and hair and paper clips, even though they are all denser than water (metal is about 8 times denser!):


When you wave a paper clip or add a drop of detergent, the paperclip sinks, although it is made of strong metal and cannot absorb any water. Both the hair and the piece of paper are dense enough to sink already… but they sink only when the surface tension is disturbed.
Explaining the Float Test
In undamaged hair, there is an oily layer that covers the surface of each cuticle scale (F layer). But when the surface of the hair is exposed, this layer is removed, leaving a hydrophilic surface.
When the surface of the damaged hair comes into contact with water, it holds hands with the surface of the water through hydrogen bonds. This means that there are fewer extra hands forming a strong “skin”, so the hair sinks.
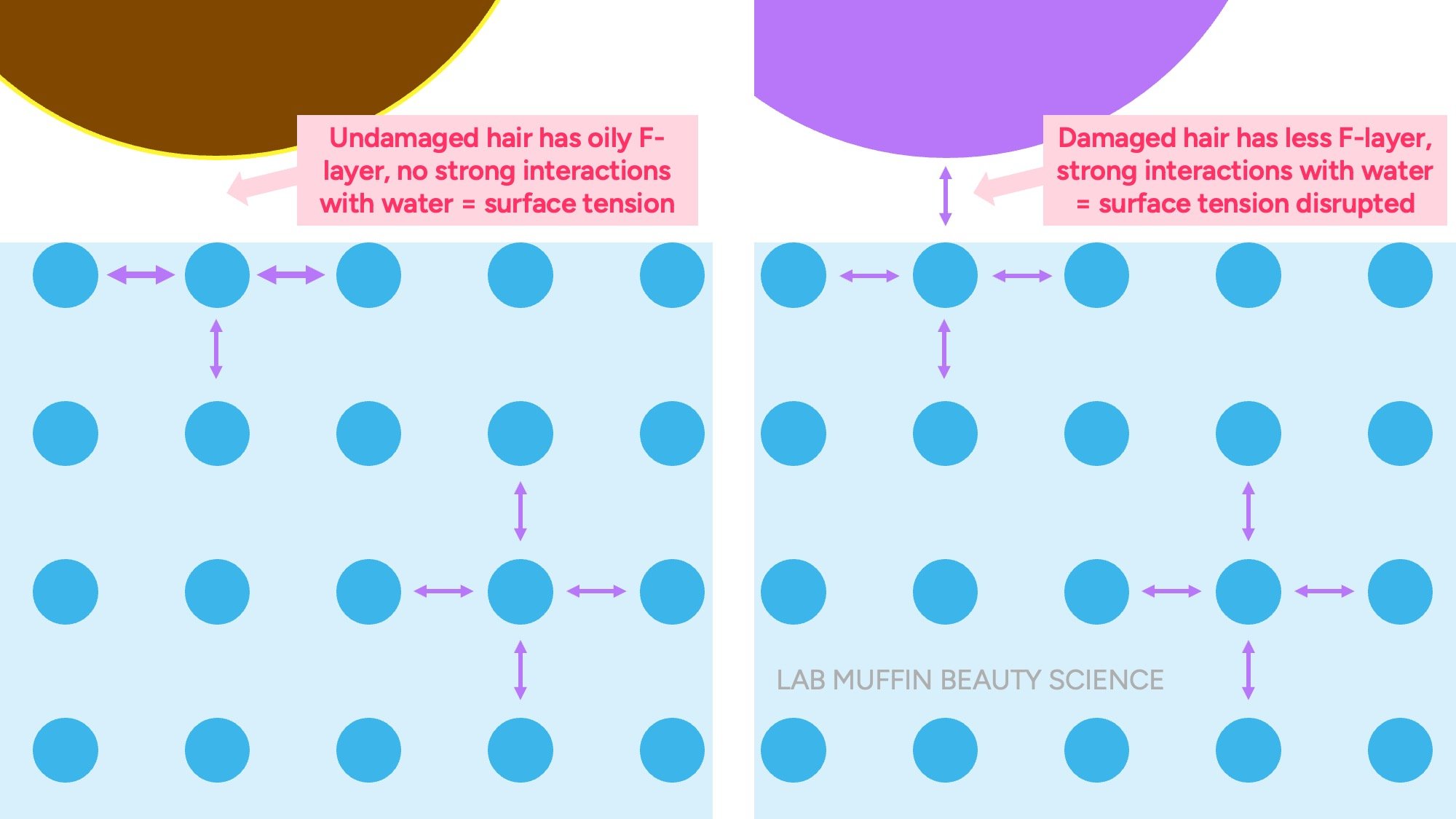

Explaining Drop Test
Surface hardness also defines the drop test.
Damaged hair can absorb somewhere around 45% of its weight in water, which is no more than 30% of undamaged hair. So the difference is not because the water penetrates the damaged hair – the water actually spreads across the surface of the hair and between them.
So how does this 30% water weight get into undamaged hair? It is because this water is not liquid water – it is gas.
Remember from high school science that in electricity, particles do not hold hands. In humid air, individual water molecules float – these are small enough to move between the scales of the cuticle inside the hair, without surface tension interfering.
Why does the “porosity” advice work?
These tests actually assess the damage on the surface of the hair – more surface damage is found as “high porosity”, and less surface damage is found as “low porosity”. Hair care based on surface damage makes a lot of sense!
But since these tests don’t measure porosity directly, it means some conclusions don’t make much sense. For example, chemical treatments may take longer to soak into your hair, but these tests say your hair has “high porosity” because you have more damage in the area. Or someone may use too much oil on their hair which leads to a “weak” effect.
Most of the time this doesn’t make a big difference, but if you are a hairdresser – please don’t use these tests to judge how long you need chemotherapy! The best test is to apply the product directly to a strand of human hair.
References
Robbins CR. Chemical and Physical Behavior of Human Hair. 5th ed. Springer Berlin Heidelberg 2012.
La Torre C, Bhushan B. Nanotribological effects of silicone type, silicone deposition rate, and surfactant type on human hair using atomic force microscopy. J Cosmet Sci. 2006;57(1):37-56.



